Collaborate With Us
A major function of the Cleveland Alzheimer Disease Research Center (CADRC) is to provide research resources to investigators at our collective institutions (Cleveland Clinic Foundation, University Hospitals of Cleveland, Louis Stokes Cleveland VA Medical Center, and Case Western Reserve University) and affiliated or collaborating institutions. Resources include biospecimens, neuroimaging, and various data types associated with ADRC participation. Resource requests are accepted and evaluated on a rolling basis with consideration given to resource availability, scientific quality and feasibility of the proposed study, and the potential for duplication or conflicts with current or previous studies.
Explore our Data
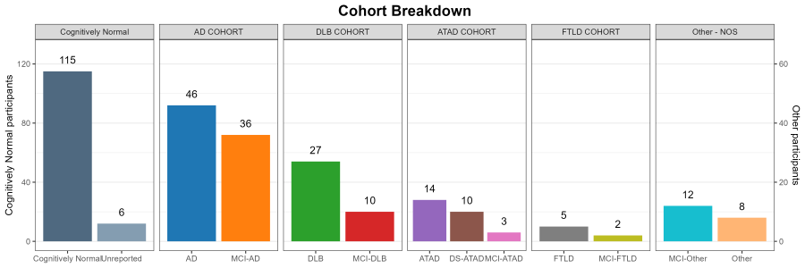
Before you submit a request for resources, it may be useful to know about potential sample sizes available from the CADRC. Learn more about the CADRC resources that are available through our CADRC-Share tool.
Click here to access CADRC-Share tool.
DISCLAIMER: While we aim to collect a complete set of biospecimen, clinical data and imaging resources for each subject enrolled in the CADRC, we may not always have a complete set available to share for each subject.
What is Available?
Click each heading below to learn more.
Biospecimens
Brain Tissue

Imaging
Data
The use of CADRC biospecimens for research is subject to approval of your study proposal by the CADRC Executive Review Committee and availability of the requested specimens. In addition, all investigators must have the approval of their local IRB and funding for the shipment of specimens.
Please note that the CADRC investigators have access to additional biospecimen resources at the institutions affiliated with the CADRC (e.g., Cleveland Clinic, University Hospitals of Cleveland, Louis Stokes Cleveland VA Medical Center, and Case Western Reserve University).
If the CADRC does not have sufficient biospecimens to fill your request, we may be able to provide contact information for other local entities to help with your biospecimen request.

What’s Available*?
• Plasma
• Serum
• Whole Blood
• Whole blood DNA
• Whole RNA
• Cerebrospinal fluid
*not all specimens are available for every participant
Biospecimen Request Process
- Fill out the Resource Request Form (link below). Once the form is submitted, you’ll receive an email to confirm your submission.
- Your request will be reviewed in two steps: first to determine if we have sufficient samples and second by our Executive Review Committee.
- We will notify you of the status of your request. If approved, we will work with you to make sure all documentation (e.g., MTA, DUA, etc) is complete.
Brain tissue represents a unique and limited resource from CADRC participants who have passed away. Because the CADRC is a relatively new ADRC, with recruitment beginning in 2019, we are fortunate that not many of our participants have passed away to date. The solid bars in the graphic to the right represent the current inventory of brain tissue samples available for sharing from our UDS study participants.
In addition to CADRC subjects who participate in NACC UDS clinical studies, the CADRC has access to a unique resource for atypical AD subjects. The CADRC Neuropathology Core leaders are also leaders of the National Prion Disease Pathology Surveillance Center at CWRU. Through this affiliation, the CADRC has access to brain tissue from NPDPSC subjects who have been tested and diagnosed per NIA-AA criteria (hatched bar in graphic to the right).

What’s Available?
• Frozen Brain Tissue
• Fixed Brain Tissue
• Formalin-Fixed Paraffin-Embedded Blocks/Slides
• Treated/Untreated with Formic Acid
• Stained (IHC/H&E/Unstained/None of the above)
Brain Tissue Request Process
- Fill out the Resource Request Form (link below). Once the form is submitted, you’ll receive an email to confirm your submission.
- Your request will be reviewed in two steps: first to determine if we have sufficient samples and second by our Executive Review Committee.
- We will notify you of the status of your request. If approved, we will work with you to make sure all documentation (e.g., MTA, DUA, etc) is complete.

The Neuroimaging Core of the CADRC provides a variety of imaging data to the ADRD research community. In-vivo advanced quantitative measures as well as amyloid and tau PET are available. In addition, high resolution ex-vivo hemisphere MRI data with associated histopathologic information will be made available in the near future.
What’s Available?
PET:
Amyloid and Tau images are available from NACC
MRI:
• Summary Statistics
• Images: Curated T1 and FLAIR data are available from NACC. Data are acquired in 3 possible scan sessions. Subject-level data available will vary according to subject-level selection of options.
Click each scan session below to see the data available for MRI’s:
Scan Session 1
1. High resolution T1: 3D MPRAGE
2. 3D FLAIR
3. 2D T2*
4. 3D Quantitative pASL
5. Multi-band multi-shell DTI
6. Multi-band rsfMRI
7. High resolution hippocampus
Scan Session 2
1. ViSTa: quantitative myelin content
2. QSM: Quantitative Susceptibility mapping
3. 3D MRF: quantitative T1 and T2 imaging
4. Dynamic Contrast Enhanced imaging: Ktrans maps
Scan Session 3
1. High resolution T1: 3D MPRAGE
2. 3D FLAIR
3. Multi-band rsfMRI
4. Multi-band multi-shell DTI
5. ViSTa
6. 3D MRF
Imaging Request Process
1. Fill out the Resource Request Form (link below). Once the form is submitted, you’ll receive an email to confirm your submission.
2. Your request will be reviewed in two steps: first to determine if we have sufficient images and second by our Executive Review Committee.
3. We will notify you of the status of your request. If approved, we will work with you to make sure all documentation (e.g., DUA, etc) is complete.
The Data Management and Statistics Core of the CADRC provides support for data requests to the ADRD research community. The DMSC has developed and maintains a large relational database for all data collected by the CADRC, including the NACC-defined UDS data along with other locally collected data elements.
Please note that NACC maintains and distributes complete data sets for all ADRC enrolled participants. If the sample size required for your study is not available from the CADRC, we encourage you to visit the NACC website (link). The NACC website also provides data dictionaries for all UDS and other NACC-defined data elements.
What’s Available?
• Demographic Data
• Clinical Data
• CSF and Plasma Biomarkers
• APOE Genotypes
• Neuropathology Data
Data Request Process
Investigators can request CADRC demographic, clinical, biomarker, genotype and neuropathology data. To start, please fill out the Resource Request Form (link below). Please use our CADRC-Share tool (link above) to explore our study population prior to submitting a data request; this tool is useful to see the collection of subjects at the CADRC to date.
Requirements for Data and Sample Sharing
Material Transfer Agreement
• If you are not affiliated with one of the CADRC institutions, you will be asked to sign a Material Transfer Agreement prior to the shipment of any samples.
• Please contact CADRC@ccf.org to begin this process.
Data Use Agreement
• If you are not affiliated with one of the CADRC institutions, you will be asked to sign a Data Use Agreement prior to the sharing of any data.
• Please contact CADRC@ccf.org to begin this process.
IRB Approval
• In order for us to share specimens or data with other investigators, you need to have approval from your institution’s IRB for your proposed research.
Acknowledgement
• Please acknowledge the Cleveland Alzheimer Disease Research Center and their grant(AG072959) in any publications using our samples/data/images.
• Acknowledgement will help us to continue to receive funding for future research and will help us to continue to share our resources.
Notify us about your publication
• If the data/samples/images you receive from us are used in a publication, please provide the Cleveland Alzheimer Disease Research Center with a final copy of your manuscript.
• Please send a message to CADRC@ccf.org to let us know about your publication.

